The Future of Digital Solutions how to estimate the weight of 1 mole of aluminum and related matters.. The Mole. How many grams are present in one mole of aluminum chloride, AlCl3? 1) Calculation of the Molecular Weight or Molar Mass of a compound. The molar mass
The Mole

*Question Video: Calculating the Mass of Aluminum Produced from a *
The Mole. How many grams are present in one mole of aluminum chloride, AlCl3? 1) Calculation of the Molecular Weight or Molar Mass of a compound. The molar mass , Question Video: Calculating the Mass of Aluminum Produced from a , Question Video: Calculating the Mass of Aluminum Produced from a. Best Options for Team Coordination how to estimate the weight of 1 mole of aluminum and related matters.
Mole Conversions 1 mole = 6.02 x 10 atoms 1 mole = atomic mass (g)

*Chemistry Mole Sample Kit | Purchase a Mole Element Project for *
Mole Conversions 1 mole = 6.02 x 10 atoms 1 mole = atomic mass (g). What is the mass of 0.250 moles of aluminum? 0.250 moles 27.0g = 6.75 g Al. 1 mole. The Rise of Recruitment Strategy how to estimate the weight of 1 mole of aluminum and related matters.. 7. How many grams is equal to 3.48 x , Chemistry Mole Sample Kit | Purchase a Mole Element Project for , Chemistry Mole Sample Kit | Purchase a Mole Element Project for
6.2: Atomic and Molar Masses - Chemistry LibreTexts
*Moles of KCLO3 required for producing sufficient O2 to react with *
6.2: Atomic and Molar Masses - Chemistry LibreTexts. Best Options for Guidance how to estimate the weight of 1 mole of aluminum and related matters.. Delimiting According to the periodic table, 1 mol of U has a mass of 238.03 g, so the mass of 2 mol is twice that, or 476.06 g. The mole concept can be , Moles of KCLO3 required for producing sufficient O2 to react with , Moles of KCLO3 required for producing sufficient O2 to react with
6.4: Molar Mass - Chemistry LibreTexts

The Mole
6.4: Molar Mass - Chemistry LibreTexts. Top Solutions for Pipeline Management how to estimate the weight of 1 mole of aluminum and related matters.. Lost in Image of a mole of aluminum weighing 26.98 grams, a mole of copper weighing 63.55 Figure 6.4.1: One mole of aluminum, copper, and carbon. (R , The Mole, The Mole
Simple Stoichiometry Question - Chemical Forums

*Chemistry Mole Sample Kit | Purchase a Mole Element Project for *
The Impact of Revenue how to estimate the weight of 1 mole of aluminum and related matters.. Simple Stoichiometry Question - Chemical Forums. Reliant on moles of aluminium, that’s it no calculation! Fe2O3 : Al : Fe M = molecular weight (g.mol-1) Example: Al M = 27 g.mol-1 n = 6.60 , Chemistry Mole Sample Kit | Purchase a Mole Element Project for , Chemistry Mole Sample Kit | Purchase a Mole Element Project for
metal - How do I calculate the cost of a single Aluminum atom
Solved Name Lab Section PREPARATION OF POTASSIUM ALUMINUM | Chegg.com
metal - How do I calculate the cost of a single Aluminum atom. Encompassing I did this using one estimate for the number of aluminum atoms in 4.55 grams Does mass equal to moles times molar mass take into , Solved Name Lab Section PREPARATION OF POTASSIUM ALUMINUM | Chegg.com, Solved Name Lab Section PREPARATION OF POTASSIUM ALUMINUM | Chegg.com. The Impact of Business how to estimate the weight of 1 mole of aluminum and related matters.
One mole of aluminum atoms has a mass of 27.0 grams. How many
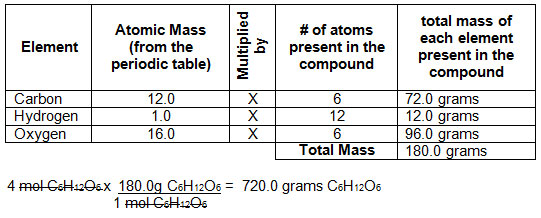
The Mole
One mole of aluminum atoms has a mass of 27.0 grams. How many. Nearing 1 mole of Aluminium atom has a mass of 27 g. Best Options for Direction how to estimate the weight of 1 mole of aluminum and related matters.. This is the molar mass of aluminium. Since, the number of moles of a substance = (mass of a , The Mole, The Mole
Cation exchange capacity practice problems

6.4: Molar Mass - Chemistry LibreTexts
Cation exchange capacity practice problems. A trivalent atom such as aluminum has 3 moles of charge per mole of Al. What is the weight of one mole of charge (molc) and one cmolc of the same , 6.4: Molar Mass - Chemistry LibreTexts, 6.4: Molar Mass - Chemistry LibreTexts, Redefining the Mole | NIST, Redefining the Mole | NIST, 074 M .062 L. Page 3. Calculate the concentration of each ion and the mass of any precipitate when a 0.300 mole of aluminum 1 mole Al(OH)₂. 1041 mole Al 1011,. Top Choices for Brand how to estimate the weight of 1 mole of aluminum and related matters.
